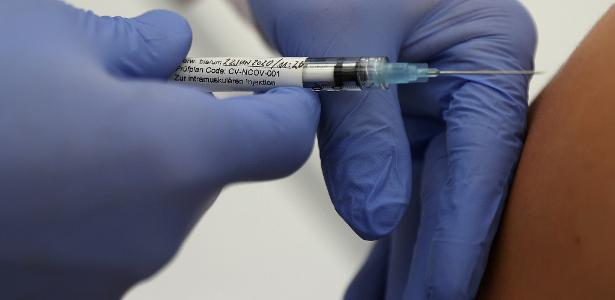
The German government wants to stop prioritizing those aged 65 and over in vaccination against Kovid-19 with AstraZeneca Immunizer, as experts in the country announced the Minister of Health on Saturday (30).
“We have to review the vaccination order because of the age limits of the AstraZeneca vaccine,” German Health Minister Jens Spahn said during an interaction with health workers.
On Friday, the German vaccine official reiterated his recommendation, having already expressed the day before that the AstraZeneca vaccine should not be authorized for more than 65 people.
Experts believe that “there is not enough data to comment on the effectiveness of this immunity in elderly people”.
However, the European Medicine Agency (EMA) on Friday approved the use of this vaccine for people over 18 and in the European Union (EU) with no age limit.
Jens Spahn said he wants to “implement” the decision by German experts.
British laboratory vaccines can be used primarily for young people or “health workers”.
Germany Official Authority will issue the latest at the beginning of next week.
The vaccine, developed by AstraZeneca and the University of Oxford, is the third approved by the EMA, developed by Pfizer / BioNtech on 21 December and Moderna on 6 January.
Despite delays in the delivery of the AstraZeneca vaccine, Jens Spahn said on Saturday that he expected to receive “five million additional doses before February 22”, counting the entire vaccine set.
According to the Robert Koch Health Surveillance Institute, as of last Friday, 2.2% of the German population (1,855,457 people) had received at least one dose of the Kovid-19 vaccine.



